Dr. Vik Kapil (top left) and Dr. Ruth Karron (top right) joined Dunwoody Mayor Lynn Deutsch (center) to discuss COVID-19 vaccine safety and development Tuesday night.
By Zachary Hansen
The COVID-19 vaccine now being distributed in Georgia is safe and effective, health experts told hundreds of Dunwoody residents Tuesday night.
The experts, Dr. Ruth Karron and Dr. Vik Kapil, fielded questions from Dunwoody Mayor Lynn Deutsch and the community during a virtual discussion. The topics focused on how vaccines are developed, Georgia’s rollout plan and when different groups of people will be able to get vaccinated.
“Part of how we come out of this pandemic is with vaccinations,” Deutsch told The Atlanta Journal-Constitution. “I heard Dr. Karron speak a few weeks ago, and it make it much easier to understand. How did this happen in 10 months? Does that mean it’s safe? She answered a lot of those questions, and I wanted the general public to have the same opportunity.”
With coronavirus cases and hospitalizations increasing across Georgia, more than 750 people tuned in to the event to ask questions about the vaccine’s safety. Both doctors emphasized the importance of getting vaccinated, with only a few exceptions.
“This is a tremendous opportunity for us to address this public health crisis,” Kapil, Georgia Department of Public Health Medical Director and Director of Health Protection, said. “We want as many people as possible to feel comfortable taking the vaccine.”
How was the vaccine developed?
The U.S. Food and Drug Administration recently approved the Pfizer vaccine last week, which is taken in two doses. The second dose is taken 21 days after the first one.
Karron, Director of the Center for Immunization Research at the John Hopkins Bloomberg School of Health, said the vaccine has shown to be roughly 95% effective at preventing COVID-19 infection among large test groups. Each clinical trial had between 30,000 and 45,000 participants, which is five to 10 times the normal amount for vaccine trials, she said.
“It doesn’t get better than that (95%) for a vaccine. It’s really quite remarkable,” Karron said, adding that early targets for the vaccine were around 50%. “This efficacy was shown across age groups, so in both the elderly and the young and also across racial and ethnic groups.”
Half of medical trial participants received the vaccine while the other half received a saltwater placebo to act as a control group. Vaccine trials are conducted double blind, meaning that neither study investigators or participants know whether they are receiving the vaccine or a placebo. About five months of data was collected before the Pfizer vaccine received an Emergency Use Authorization from the FDA.
She said one important thing the vaccine trials do not test for are transmissibility, so it’s unclear whether those who receive the vaccine can infect those who are unvaccinated.
“They (trials) will tell us whether a vaccine will keep you from getting sick, either mildly ill or seriously ill.” Karron said. “But it won’t tell you if the vaccine will stop you from giving it to your friend, your family member or your neighbor.”
How does the vaccine work?
Karron said three types of vaccines are currently in development. The Pfizer vaccine, along with the Moderna vaccine that is being considered for FDA approval Thursday, are mRNA vaccines.
An mRNA vaccine contains material from the COVID-19 virus, and those materials instruct our cells on how to make a harmless protein that is unique to the virus, she said. Our natural defense against disease, white blood cells, realize the proteins should not be there and destroy them. As a result, our bodies more quickly recognize COVID-19 if infection were to occur and can fight the virus much more efficiently, Karron said.
She said the vaccine does not contain the “live” virus, so COVID-19 symptoms are not side effects. However, she did say some trial patients reported mild symptoms after receiving the vaccine, comparing it to the side effects of the Shingles vaccine.
“That’s just something people need to prepare for and be aware of,” she said, adding that people should consult their physician or allergist before taking the vaccine if they have unique health conditions or risks.
She said it is too early to tell whether the vaccine will eventually be administered annually like the flu vaccine.
“So far with this virus, we don’t have evidence of that kind of the chance, the kind of change we see with the flu,” she said. “So time will tell.”
Karron added that those who were previously infected with COVID-19 should still receive the vaccine, since it is more effective than the lingering antibodies from fighting the natural virus.
When can I receive the vaccine?
Distribution of the vaccine is left up to each state, Kapil said. At the moment, Georgia is expected to receive nearly 85,000 doses of the Pfizer vaccine, which will first be made available for medical workers and long-term case facility staff and residents.
Georgia’s rollout plan includes four phases, and we’re in the first section of phase 1, Kapil said. A vaccine has not been approved for children yet.
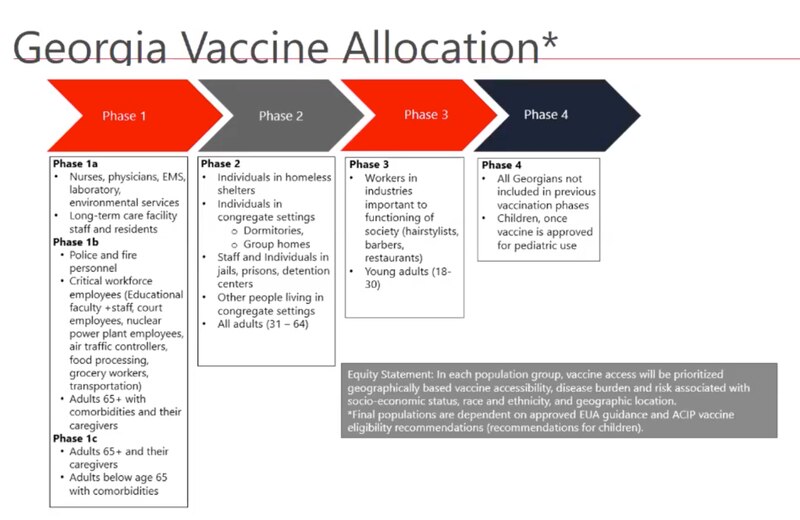
This is a chart of the four phases of vaccine availability in Georgia.
Kapil said it’s too early to know when the state will move onto the next phase, since there are still questions about supply, demand and whether more vaccines become FDA approved.
“For the individual receiving the vaccine, in almost all cases it would be free of charge,” he said, adding that some insurance providers may bill for minor administrative costs. Those who are uninsured will be covered by a federal Health Resources and Services Administration program.
Regardless of vaccine availability, he said it will be awhile before residents should throw away their face masks.
“It is very, very important to understand that it (the vaccines) are just one important tool in dealing with this public health crisis and pandemic,” he said. “We have to remain very diligent to use all of those tools, including those basic public health safety measures.”
Karron echoed that residents need to stay vigilant, since it will take awhile for majority of the population to receive the vaccine.
“This is not a time for us to let down our guard,” she said. “Until most of our population is vaccinated, we will not have the kind of herd immunity that will allow us to stop these medical measures.”
Dunwoody’s mayor said she plans to get vaccinated once it’s made available to her.
Read the original story on AJC.com.











